A Deep Dive into the Periodic Table
2024-07-26
The periodic table is a tabular arrangement of chemical elements, organized by atomic number, electron configuration, and recurring chemical properties. As its name suggests, the table's arrangement reveals periodic trends in elemental properties. The rows of the table are called periods, and the columns are called groups. Generally, within the same period, metallic elements are on the left, and non-metallic elements are on the right. Elements in the same group share similar chemical properties. Some groups have specific names, including Group 1 (IA) known as the alkali metals, Group 2 (IIA) as the alkaline earth metals, Group 17 (VIIA) as the halogens, and Group 18 (VIIIA) as the noble gases.
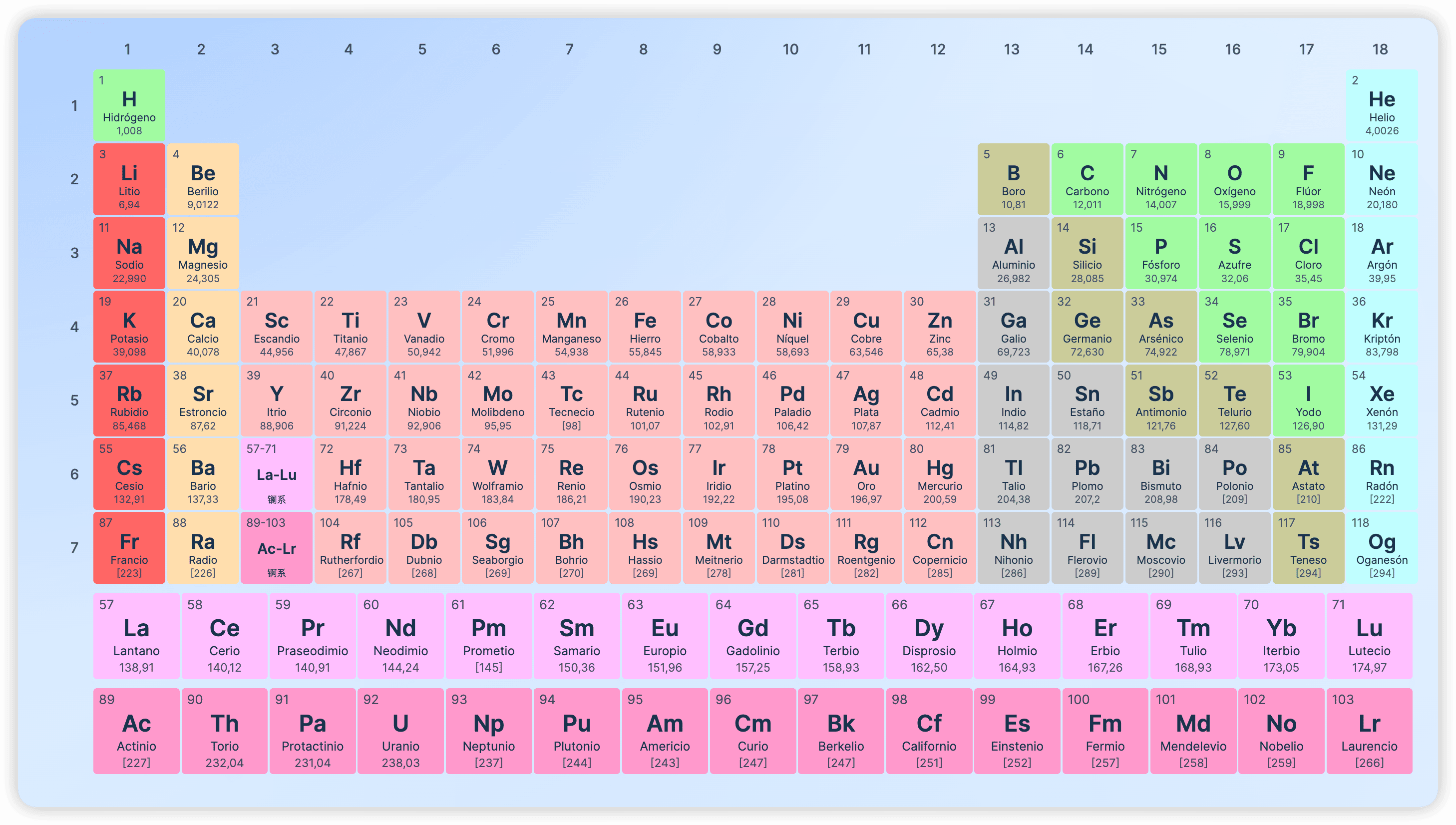
The periodic trends in the table can be used to deduce relationships between the properties of different elements and to predict the properties of undiscovered or newly synthesized elements. The periodic table was first published by the Russian chemist Dmitri Mendeleev in 1869, primarily to show the periodic patterns among the known elements of his time. However, he also successfully used it to predict some properties of then-unknown elements that filled the gaps in his table. With the discovery of new elements and the development of theoretical models of chemical properties, Mendeleev's ideas have been continuously refined. The modern periodic table not only provides a useful framework for analyzing chemical reactions but is also widely used in other fields of chemistry and even nuclear physics.
From element 1 (hydrogen) to element 118 (Oganesson), all have been discovered or successfully synthesized, filling the first seven periods of the periodic table. However, only the first 94 elements occur naturally on Earth, and some only in trace amounts; elements 95 and beyond are synthesized in laboratories or nuclear reactors. The next synthesized element will begin the eighth period of the table, and thus, significant efforts are being directed towards this goal, with theories already pointing to possible new elements. Furthermore, new radioactive isotopes of various elements are continuously being synthesized in laboratories around the world.